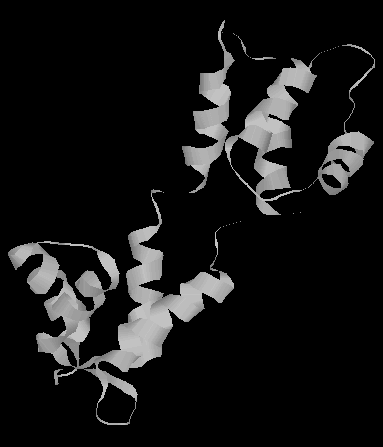
Calmodulin has two globular domains connected by a single alpha helix (see picture below). Each globular domain contains 2 Ca2+ binding sites. Calmodulin undergoes large conformational changes when bound to Ca2+. Also, when bound to myosin, the globular domains remain relatively unchanged, but the alpha helix connecting them unwound and contains a sharp bend.
Calcium-free Calmodulin:

Calmodulin bound with Calcium:
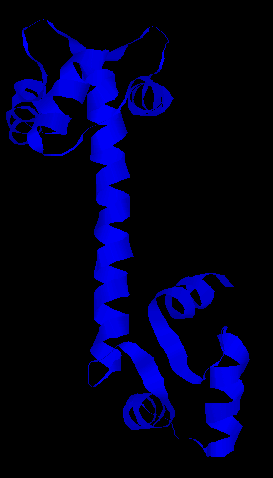
Calmodulin complexed with Rabbit Myosin Light Chain Kinase:
 |
 |
Last updated: 7/13/01