 |
 |
 |
Proteins may make "bad" connections when trying to fold to their native state. These "bad" connectections, or aggregates, can cause the protein to function improperly. Chaperones can prevent and reverse such "bad" connections by binding to and releasing the unfolded or aggregated protein during the folding process. Chaperones do not increase the rate of protein folding, they only increase its efficiency.
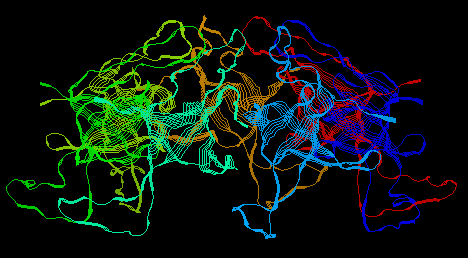
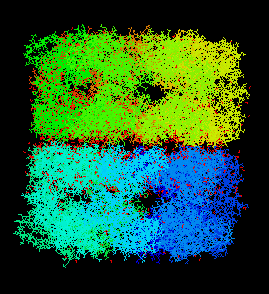
GroEL and GroES work together (interact) to help peoteins fold into their native state properly. They do this by surrounding the protein like a cage thereby providing a safe environment for the protein. GroES binds to the top of GroEL and forms a cage around the protein. During this interaction, GroEL undergoes large conformational changes. Pictures of these proteins (obtained from the Protein Data Bank and viewed through RasMol) are shown below.
GroEL/GroES Complex:
 |
 |
 |
GroES, Alone:
 |
 |
GroEL:
 |
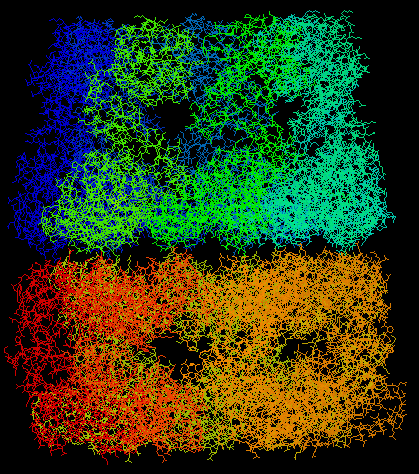 |
Last updated: 7/13/01